aromano
New Member
I was wondering where my high calcium values were coming from, as I tested the water 24 hours after a 10 gallon water change and surprisingly the CA values increased significantly.
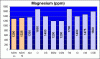
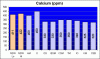
I decided then to test the water from a 5 gallon bucket with new water mixed with Oceanic Salt Mix and discovered that at specific gravity of 1.025 the CA value tested at 620ppm. I am going to stop using this salt brand, but now I am worried about precipitation and other side effects that the high calcium can do to my tank.
I tested the water today and my results were:
PH 7.9
Ammonia 0.0 mg/l
Nitrite 0.0 mg/l
Nitrate 5.0 mg/l
Calcium 700 ppm
Alkalinity 2.51 meq/L
KH 7.0
Salinity 1.023
Temperature 78.4 – 79.3
Most of my corals seem to be doing OK, but both of my brain corals and a large colony of ricordia mushrooms don't look good lately and have been very stressed. I am almost certain that is related to this issue. I am debating between experimenting either Kent or Instant Ocean Salt Mix, which have lower calcium levels. If anyone has any comments or suggestions about this topic or how to solve this issue, please let me know.
As far as my knowledge goes, the coral's skeleton is a result of waste deposit so it can multiply its cells. This process cannot be done with calcium in them, so corals have developed a means to deal with removing the calcium from their cells... But having exceedingly high levels puts the corals in a state of stress. So the more calcium values are over natural sea water, the harder the corals have to work, consuming the energy budget they have other need things.
Here is what Oceanic is telling people
"At a specific gravity of 1.021 – 1.023 Oceanic sea salt yields calcium concentrations of 420 – 480 ppm which are comparable to natural sea water.
Some reef tank aquarists prefer to maintain their tanks at a specific gravity of 1.025 or greater. With Oceanic salt, this high specific gravity results in calcium levels that can exceed 550 ppm which can disturb the delicate carbonate/bicarbonate equilibrium causing transient pH fluctuations. For reef tanks it is recommended that Oceanic salt be used at 1.024 specific gravity which should yield high calcium and magnesium levels without causing hardness and pH imbalance.
We have talked with the scientist who formulated our salt about this issue. He does not feel the formulation should be changed. Here are some suggestions that were given from him for aquarist who feel it is necessary to run their tanks and higher specific gravity.
1) Mix Oceanic salt to sg 1.023. To this slowly add and completely dissolve solid analytical reagent grade sodium chloride to desired sg. This procedure will increase sg without effecting calcium and magnesium levels.
2) Mix Oceanic salt to sg 1.023 – 1.025. Before adding to tank, treat with dKh hardness buffer to attain dKh of 5-7. Add gradually to tank after solution has equilibrated.
If I can be of further assistance, please let me know."
I decided then to test the water from a 5 gallon bucket with new water mixed with Oceanic Salt Mix and discovered that at specific gravity of 1.025 the CA value tested at 620ppm. I am going to stop using this salt brand, but now I am worried about precipitation and other side effects that the high calcium can do to my tank.
I tested the water today and my results were:
PH 7.9
Ammonia 0.0 mg/l
Nitrite 0.0 mg/l
Nitrate 5.0 mg/l
Calcium 700 ppm
Alkalinity 2.51 meq/L
KH 7.0
Salinity 1.023
Temperature 78.4 – 79.3
Most of my corals seem to be doing OK, but both of my brain corals and a large colony of ricordia mushrooms don't look good lately and have been very stressed. I am almost certain that is related to this issue. I am debating between experimenting either Kent or Instant Ocean Salt Mix, which have lower calcium levels. If anyone has any comments or suggestions about this topic or how to solve this issue, please let me know.
As far as my knowledge goes, the coral's skeleton is a result of waste deposit so it can multiply its cells. This process cannot be done with calcium in them, so corals have developed a means to deal with removing the calcium from their cells... But having exceedingly high levels puts the corals in a state of stress. So the more calcium values are over natural sea water, the harder the corals have to work, consuming the energy budget they have other need things.
Here is what Oceanic is telling people
"At a specific gravity of 1.021 – 1.023 Oceanic sea salt yields calcium concentrations of 420 – 480 ppm which are comparable to natural sea water.
Some reef tank aquarists prefer to maintain their tanks at a specific gravity of 1.025 or greater. With Oceanic salt, this high specific gravity results in calcium levels that can exceed 550 ppm which can disturb the delicate carbonate/bicarbonate equilibrium causing transient pH fluctuations. For reef tanks it is recommended that Oceanic salt be used at 1.024 specific gravity which should yield high calcium and magnesium levels without causing hardness and pH imbalance.
We have talked with the scientist who formulated our salt about this issue. He does not feel the formulation should be changed. Here are some suggestions that were given from him for aquarist who feel it is necessary to run their tanks and higher specific gravity.
1) Mix Oceanic salt to sg 1.023. To this slowly add and completely dissolve solid analytical reagent grade sodium chloride to desired sg. This procedure will increase sg without effecting calcium and magnesium levels.
2) Mix Oceanic salt to sg 1.023 – 1.025. Before adding to tank, treat with dKh hardness buffer to attain dKh of 5-7. Add gradually to tank after solution has equilibrated.
If I can be of further assistance, please let me know."